Nembutal solution orale
$1,200.00
CLIQUEZ POUR COMMANDER SUR WHATSAPP MAINTENANT !
Télégramme:Euthanasie et sommeil
Email:euthanasiasleep@protonmail.com
Solution orale de Nembutal Dose de Lathal pour l'euthanasie
Phenobarbital Oral Solution Description
Barbiturates are nonselective central nervous system (CNS) depressants that are primarily used as sedative-hypnotics. In subhypnotic doses, they are also used as anticonvulsants. Barbiturates and their sodium salts are subject to control under the Federal Controlled Substances Act. Phenobarbital is a barbituric acid derivative and occurs as white, odorless, small crystals or crystalline powder that is very slightly soluble in water; soluble in alcohol, in ether, and solutions of fixed alkali hydroxides and carbonates; and sparingly soluble in chloroform. Phenobarbital is 5-ethyl-5-phenylbarbituric acid and has the empirical formula C 12H 12N 2O 3. Its molecular weight is 232.24. It has the following structural formula: Pentobarbital oral solution (PB) is a euthanasia drug in doses of 2 to 10 grams, causing death within 15–30 minutes. We report a case of recovery from lethal pentobarbital deliberate self-poisoning with confirmatory serum drug concentrations.
Rapport de cas
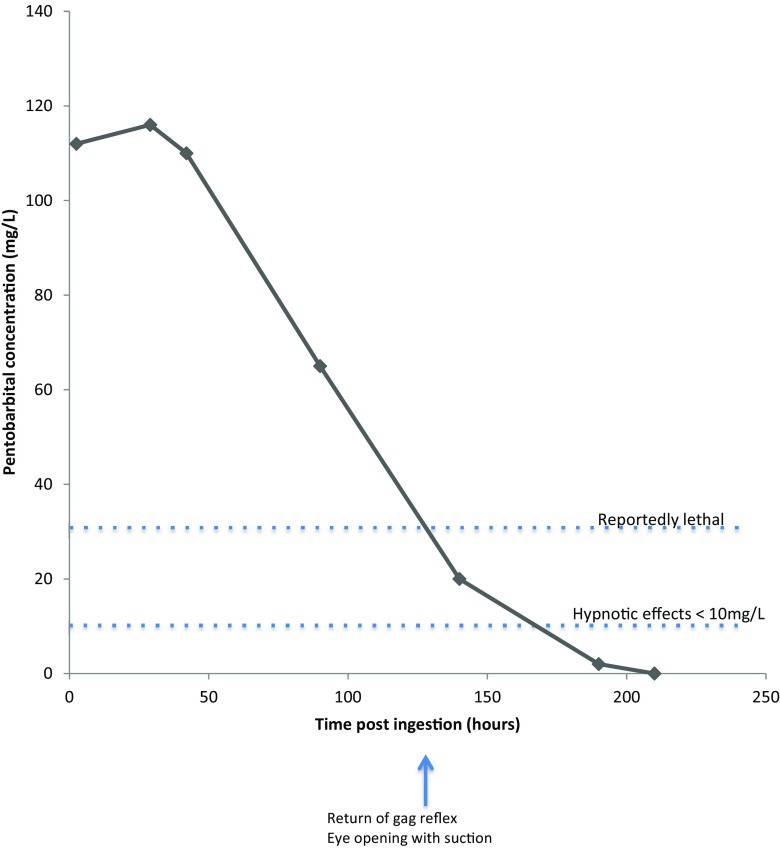
Un homme de 45 ans a acheté 20 grammes de poudre de PB sur Internet. Il a ingéré cette poudre et a alerté sa mère 10 minutes plus tard. Celle-ci l'a trouvé sans réaction et a commencé la réanimation cardio-pulmonaire (RCP). Dans les 20 minutes suivant l'ingestion, les services médicaux d'urgence sont arrivés et ont commencé les soins avancés de réanimation. À son arrivée au service des urgences, son rythme cardiaque était de 116 bpm, sa tension artérielle de 117/62 mmHg et il était sous perfusion d'adrénaline. Il était hypotonique et hypothermique, et ses réflexes du tronc cérébral étaient absents. L'ECG et le scanner cérébral étaient normaux. Du charbon actif lui a été administré et il a été admis en soins intensifs. Il est resté comateux avec absence de réflexes du tronc cérébral jusqu'au 5e jour. L'angiographie cérébrale du jour 3 était normale. Les analyses d'urine qualitatives ont détecté du pentobarbital, ce qui suggère que les effets médicamenteux en cours sont à l'origine du coma. Il a été extubé le 10e jour et s'est complètement rétabli. Deux heures et demie après l'ingestion, la concentration de PB était de 112 mg/L ; le PB a atteint un pic de 116 mg/L à 29 heures ; le PB était de 2 mg/L à 190 heures et indétectable plus de 200 heures après l'ingestion.
Discussion
La concentration moyenne de PB chez les personnes décédées serait d'environ 30 mg/L. Ce patient a survécu à des concentrations sériques plus élevées grâce à une réanimation cardio-pulmonaire précoce et à une assistance cardio-respiratoire prolongée aux soins intensifs. L'évaluation de la mort du tronc cérébral doit être reportée jusqu'à ce que le PB ait été correctement éliminé.
Le pentobarbital (Nembutal) est un sédatif-hypnotique barbiturique à courte durée d'action largement utilisé dans la pratique vétérinaire pour l'anesthésie et l'euthanasie. Il est également recommandé comme médicament pour l'euthanasie ou le suicide assisté en raison de l'apparition rapide du coma et de la perception d'une mort paisible. Le fait que les médias populaires rapportent que le pentobarbital est une méthode de suicide pacifique a suscité un intérêt accru pour son obtention dans des juridictions où il est moins réglementé [1]. Il est peu probable que des mesures de réanimation soient prises dans ces circonstances.
Nous rapportons un cas de survie à la suite d'un auto-empoisonnement délibéré avec un produit potentiellement dangereux pour la santé. dose létale de pentobarbital obtenu via Internet, chez un patient qui a regretté ses actes et a demandé de l'aide presque immédiatement. Les concentrations sériques de médicaments confirmées sont présentées dans le contexte de l'évolution clinique du patient.
Histoire de cas
A 45-year-old male impulsively ingested 20 grams of pentobarbital (Nembutal) that he had purchased via the Internet from a source overseas 2 years previously. He had a history of bipolar affective disorder, trigeminal neuralgia, and chronic pain. His regular medications included venlafaxine, gabapentin, and asenapine. He alerted his mother 10 minutes after taking the overdose. She immediately contacted emergency medical services (EMS). When she returned to him, he was on the floor and unconscious, and she immediately commenced cardiopulmonary resuscitation (CPR). EMS arrived 10 minutes after the call (approximately 20 minutes post-ingestion) and found him to be in a pulseless electrical activity cardiac arrest. CPR continued, and advanced life support (ALS) measures were commenced. He received two intravenous doses of 1 mg epinephrine during initial resuscitation, with return of spontaneous circulation (ROSC) occurring after 10 min. The patient was intubated and ventilated. He had a further brief cardiac arrest 30 minutes later, with ROSC being achieved after another 2 minutes of CPR and a further 1 mg epinephrine. He was commenced on an epinephrine infusion at 100 μg/min, given a 500-mL normal saline bolus, and transported to the emergency department (ED).
Le patient est arrivé aux urgences 95 minutes après l'appel initial aux services médicaux d'urgence. Lors de sa présentation, il était inconscient (GCS 3/15) sans sédation supplémentaire, avait des pupilles fixes dilatées, était en apnée sous respirateur et n'avait pas de réflexes au niveau du tronc cérébral. Il était en hypothermie (33,8 °C). La fréquence cardiaque était de 116 bpm et la tension artérielle de 117/62 mmHg sous perfusion d'adrénaline à 100μg/min. Les gaz du sang veineux indiquaient un pH de 7,02, une pCO2 60 mmHg, HCO3 15 mmol/L, and 11.9 mmol/L lactate. Serum ethanol, paracetamol, and salicylate were undetectable. ECG was unremarkable,e and CT brain revealed no acute abnormality. A single dose of 50 g activated charcoal was administered via NGT and he was admitted to the ICU.
Il a été décidé de lui administrer un traitement de soutien et de ne pas mettre en place de techniques d'élimination extracorporelle. Le premier jour aux soins intensifs, il a développé une polyurie, avec un débit urinaire atteignant 300 ml/h, et une hypernatrémie (Na sérique 149 mmol/L), il a donc été traité avec une dose de desmopressine.
Il a nécessité un soutien vasopresseur par perfusion de noradrénaline (dose maximale de 30 μg/min) pendant les 5 premiers jours de son admission. Il est resté comateux sans sédation, avec des réflexes du tronc cérébral absents, ce qui a suscité des discussions sur le diagnostic de mort cérébrale. Une angiographie cérébrale à quatre vaisseaux a été réalisée le 3e jour. Elle a montré une perfusion cérébrale normale. L'urine envoyée pour une analyse qualitative par chromatographie en phase gazeuse et spectrométrie de masse (GC-MS) a détecté du pentobarbital, confirmant que les effets médicamenteux en cours étaient la cause probable du coma persistant. Le cinquième jour, on a constaté un retour du réflexe nauséeux lors de l'aspiration et l'ouverture des yeux à des stimuli douloureux. Une perfusion de propofol a été mise en place pour permettre la tolérance à la sonde endotrachéale à partir du 7e jour. Cependant, l'extubation a été retardée en raison de l'apparition d'une pneumopathie d'aspiration. Il a finalement été extubé le 10e jour après le surdosage et a été renvoyé dans le service médical le lendemain. Il a dû rester 10 jours de plus à l'hôpital pour le traitement continu de sa pneumopathie d'aspiration et la physiothérapie pour le reconditionnement. Il s'est complètement rétabli sur le plan neurologique et a confirmé l'ingestion de 20 grammes de poudre de pentobarbital mélangée à de l'eau. Il a été transféré dans un établissement psychiatrique le 22e jour après l'overdose. Il y est resté hospitalisé pendant trois semaines supplémentaires avant d'être renvoyé chez lui, tout en bénéficiant d'un suivi psychiatrique ambulatoire.
Les concentrations sériques sérielles de pentobarbital ont été dosées rétrospectivement par chromatographie liquide à haute performance/spectrométrie de masse (HPLC/MS) et sont résumées dans la Fig. 1. La concentration maximale était de 116 mg/ml environ 29 heures après l'ingestion (taux thérapeutique de 1,8 à 4,7 mg/l).
Fig. 1.
Le consentement écrit pour la publication de ce cas a été obtenu et fourni au journal.
Phenobarbital Oral Solution – Clinical Pharmacology
Barbiturates are capable of producing all levels of CNS mood alteration, from excitation to mild sedation, hypnosis, and deep coma. Overdosage can produce death. In high enough therapeutic doses, barbiturates induce anesthesia.
Les barbituriques dépriment le cortex sensoriel, diminuent l'activité motrice, altèrent la fonction cérébelleuse et provoquent la somnolence, la sédation et l'hypnose.
Barbiturate-induced sleep differs from physiologic sleep. Sleep laboratory studies have demonstrated that barbiturates reduce the amount of time spent in the rapid eye movement (REM) phase of sleep or the dreaming stage. Also, in Stages III and IV, sleep is decreased. Following abrupt cessation of barbiturates used regularly, patients may experience markedly increased dreaming, nightmares, and/or insomnia. Therefore, withdrawal of a single therapeutic dose over 5 or 6 days has been recommended to lessen the REM rebound and disturbed sleep that contribute to the drug withdrawal syndrome (for example, the dose should be decreased from 3 to 2 doses/day for 1 week).
In studies, secobarbital sodium and pentobarbital sodium have been found to lose most of their effectiveness for both inducing and maintaining sleep by the end of 2 weeks of continued drug administration, even with the use of multiple doses. As with secobarbital sodium and pentobarbital sodium, other barbiturates (including amobarbital) might be expected to lose their effectiveness for inducing and maintaining sleep after about 2 weeks. The short-, intermediate-, and, to a lesser degree, long-acting barbiturates have been widely prescribed for treating insomnia. Although the clinical literature abounds with claims that the short-acting barbiturates are superior for producing sleep whereas the intermediate-acting compounds are more effective in maintaining sleep, controlled studies have failed to demonstrate these differential effects. Therefore, as sleep medications, barbiturates are of limited value beyond short-term use.
Barbiturates have little analgesic action at subanesthetic doses. Rather, in subanesthetic doses, these drugs may increase the reaction to painful stimuli. All barbiturates exhibit anticonvulsant activity in anesthetic doses. However, of the drugs in this class, only phenobarbital, mephobarbital, and metharbital are effective as oral anticonvulsants in subhypnotic doses.
Barbiturates are respiratory depressants, and the degree of respiratory depression is dependent upon the dose. With hypnotic doses, respiratory depression produced by barbiturates is similar to that which occurs during physiologic sleep and is accompanied by a slight decrease in blood pressure and heart rate.
Studies in laboratory animals have shown that barbiturates cause reductions in the tone and contractility of the uterus, ureters, and urinary bladder. However, concentrations of the drugs required to produce this effect in humans are not reached with sedative/hypnotic doses.
Barbiturates do not impair the normal hepatic function but have been shown to induce liver microsomal enzymes, thus increasing and/or altering the metabolism of barbiturates and other drugs (see Drug Interactions under PRÉCAUTIONS).
PHARMACOKINETICS
Barbiturates are absorbed in varying degrees following oral or parenteral administration. The salts are more rapidly absorbed than are the acids. The rate of absorption is increased if the sodium salt is ingested as a dilute solution or taken on an empty stomach.
La durée d'action, qui est liée à la vitesse à laquelle les barbituriques sont redistribués dans l'organisme, varie d'une personne à l'autre et chez la même personne de temps à autre.
Phenobarbital is classified as a long-acting barbiturate when taken orally. Its onset of action is 1 hour or longer, and its duration of action ranges from 10 to 12 hours.
Barbiturates are weak acids that are absorbed and rapidly distributed to all tissues and fluids, with high concentrations in the brain, liver, and kidneys. Lipid solubility of the barbiturates is the dominant factor in their distribution within the body. The more lipid soluble the barbiturate, the more rapidly it penetrates all tissues of the body. Barbiturates are bound to plasma and tissue proteins to a varying degree with the degree of binding increasing directly as a function of lipid solubility.
Phenobarbital has the lowest lipid solubility, lowest plasma binding, lowest brain protein binding, the longest delay in onset of activity, and the longest duration of action. The plasma half-life for phenobarbital in adults ranges between 53 and 118 hours with a mean of 79 hours. The plasma half-life for phenobarbital in children and newborns (less than 48 hours old) ranges between 60 to 180 hours with a mean of 110 hours.
Barbiturates are metabolized primarily by the hepatic microsomal enzyme system, and the metabolic products are excreted in the urine, and, less commonly, in the feces. Approximately 25% to 50% of a dose of phenobarbital is eliminated unchanged in the urine. The excretion of unmetabolized barbiturate is one feature that distinguishes the long-acting category from those belonging to other categories, which are almost entirely metabolized. The inactive metabolites of the barbiturates are excreted as conjugates of glucuronic acid.
Indications and Usage for Nembutal Oral Solution
A. Sedative
B. Anticonvulsant – For the treatment of generalized and partial seizures.
Contraindications
Phenobarbital is contraindicated in patients who are hypersensitive to barbiturates, in patients with a history of manifest or latent porphyria, and in patients with marked impairment of liver function or respiratory disease in which dyspnea or obstruction is evident.
Warnings
1. Habit Forming
Phenobarbital may be habit-forming. Tolerance and psychological and physical dependence may occur with continued use (see DRUG ABUSE AND DEPENDENCE et Pharmacokinetics under LA PHARMACOLOGIE CLINIQUE). Patients who have psychological dependence on barbiturates may increase the dosage or decrease the dosage interval without consulting a physician and may subsequently develop a physical dependence on barbiturates. In order to minimize the possibility of overdosage or the development of dependence, the prescribing and dispensing of sedative-hypnotic barbiturates should be limited to the amount required for the interval until the next appointment. Abrupt cessation after prolonged use in a person who is dependent on the drug may result in withdrawal symptoms, including delirium, convulsions, and possibly death. Barbiturates should be withdrawn gradually from any patient known to be taking excessive doses over long periods of time (see L'ABUS DE DROGUES ET LA DÉPENDANCE).
2. Acute or Chronic Pain
Caution should be exercised when barbiturates are administered to patients with acute or chronic pain because paradoxical excitement could be induced or important symptoms could be masked. However, the use of barbiturates as sedatives in the postoperative surgical period and as adjuncts to cancer chemotherapy is well established.
3. Usage in Pregnancy
Barbiturates can cause fetal damage when administered to a pregnant woman. Retrospective, case-controlled studies have suggested a connection between the maternal consumption of barbiturates and a higher-than-expected incidence of fetal abnormalities. Barbiturates readily cross the placental barrier and are distributed throughout fetal tissues; the highest concentrations are found in the placenta, fetal liver, and brain. Fetal blood levels approach maternal blood levels following parenteral administration.
Withdrawal symptoms occur in infants born to women who receive barbiturates throughout the last trimester of pregnancy (see L'ABUS DE DROGUES ET LA DÉPENDANCE).
If Phenobarbital is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
4. Usage in Children
Phenobarbital has been reported to be associated with cognitive deficits in children taking it for complicated febrile seizures.
5. Synergistic Effects
Precaution
Barbiturates may be habit-forming. Tolerance and psychological and physical dependence may occur with continued use (see L'ABUS DE DROGUES ET LA DÉPENDANCE).
Barbiturates should be administered with caution, if at all, to patients who are mentally depressed, have suicidal tendencies, or have a history of drug abuse.
Elderly or debilitated patients may react to barbiturates with marked excitement, depression, or confusion. In some persons, especially children, barbiturates repeatedly produce excitement rather than depression.
Chez les patients présentant une atteinte hépatique, les barbituriques doivent être administrés avec prudence et, dans un premier temps, à des doses réduites. Les barbituriques ne doivent pas être administrés aux patients présentant des signes prémonitoires de coma hépatique.
The systemic effects of exogenous and endogenous corticosteroids may be diminished by Phenobarbital. Thus, this product should be administered with caution to patients with borderline hypoadrenal function, regardless of whether it is of pituitary or of primary adrenal origin.
Information for Patients
The following information and instructions should be given to patients receiving barbiturates.
1. The use of barbiturates carries with it an associated risk of psychological and/or physical dependence. The patient should be warned against increasing the dose of the drug without consulting a physician.
2. Barbiturates may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patients should be cautioned accordingly.
3. Alcohol should not be consumed while taking barbiturates. The concurrent use of barbiturates with other CNS depressants (eg., alcohol, narcotics, tranquilizers, and antihistamines) may result in additional CNS-depressant effects.
Références
- 1.Cantrell FL, Nordt S, McIntyre I, Schneir A. Death on the doortep of a border community-intentional self-poisoning with veterinary pentobarbital. Clin Toxicol. 2010;48:849-850. doi : 10.3109/15563650.2010.512562. [DOI] [PubMed] [Google Scholar]
- 2.Bosshard G, Ulrich E, Bar W. 748 cas de suicide assistés par une organisation suisse de droit à mourir. Swiss Med Wkly. 2003;133:310-317. doi : 10.4414/smw.2003.10212. [DOI] [PubMed] [Google Scholar]
- 3.Mactier R, Laliberte M, Mardini J, Ghannoum M, Lavergne V, et al. Extracorporeal treatment for barbiturate poisoning : recommendations from the EXTRIP Workgroup. Am J Kidney Dis. 2014;64:347-358. doi : 10.1053/j.ajkd.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Roberts DM, Buckley NA. Enhanced elimination in acute barbiturate poisoning-a systematic review. Clin Toxicol. 2011;49:2-12. doi : 10.3109/15563650.2010.550582. [DOI] [PubMed] [Google Scholar]
- 5.Heinemeyer G, Roots I, Dernhardt R. Monitoring of pentobarbital plasma levels in critical care patients suffering from increased intracranial pressure. Ther Drug Monit. 1986;8:145-150. doi : 10.1097/00007691-198606000-00003. [DOI] [PubMed] [Google Scholar]
- 6.McCarron MM, Schulze BW, Walberg CB, Thompson GA, Ansari A. Short acting barbiturate overdosage. Correlation of intoxication score with serum barbiturate concentration. JAMA. 1982;248:55-61. doi : 10.1001/jama.1982.03330010029026. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan R, Hodgman MJ, Kao L, Tormoehlen LM. Baclofen overdose mimicking brain death (overdose de baclofène imitant la mort cérébrale). Clin Toxicol. 2012;50:141-144. doi : 10.3109/15563650.2011.654209. [DOI] [PubMed] [Google Scholar]
- 8.Neavyn MJ, Stolbach A, Greer DM, Nelson LS, Smith SW, et al. ACMT position statement : determining brain death in adults after drug overdose. J Med Toxicol. 2017;13:271-273. doi : 10.1007/s13181-017-0606-8. [DOI] [Article gratuit PMC] [PubMed] [Google Scholar]
Soyez le premier à laisser votre avis sur “Nembutal oral solution” Annuler la réponse
Produits similaires
Nembutal
Nembutal










Avis
Il n’y a pas encore d’avis.